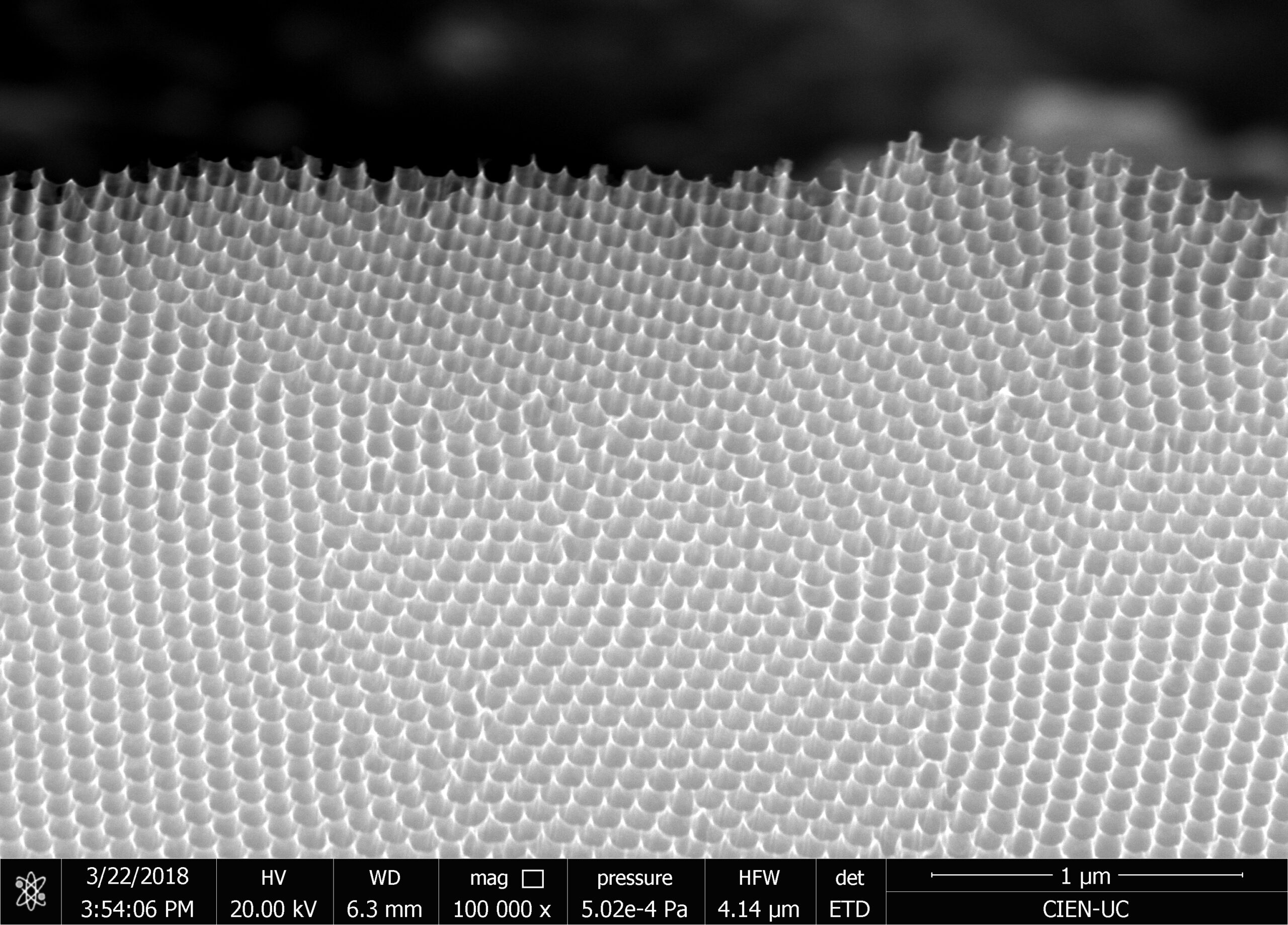
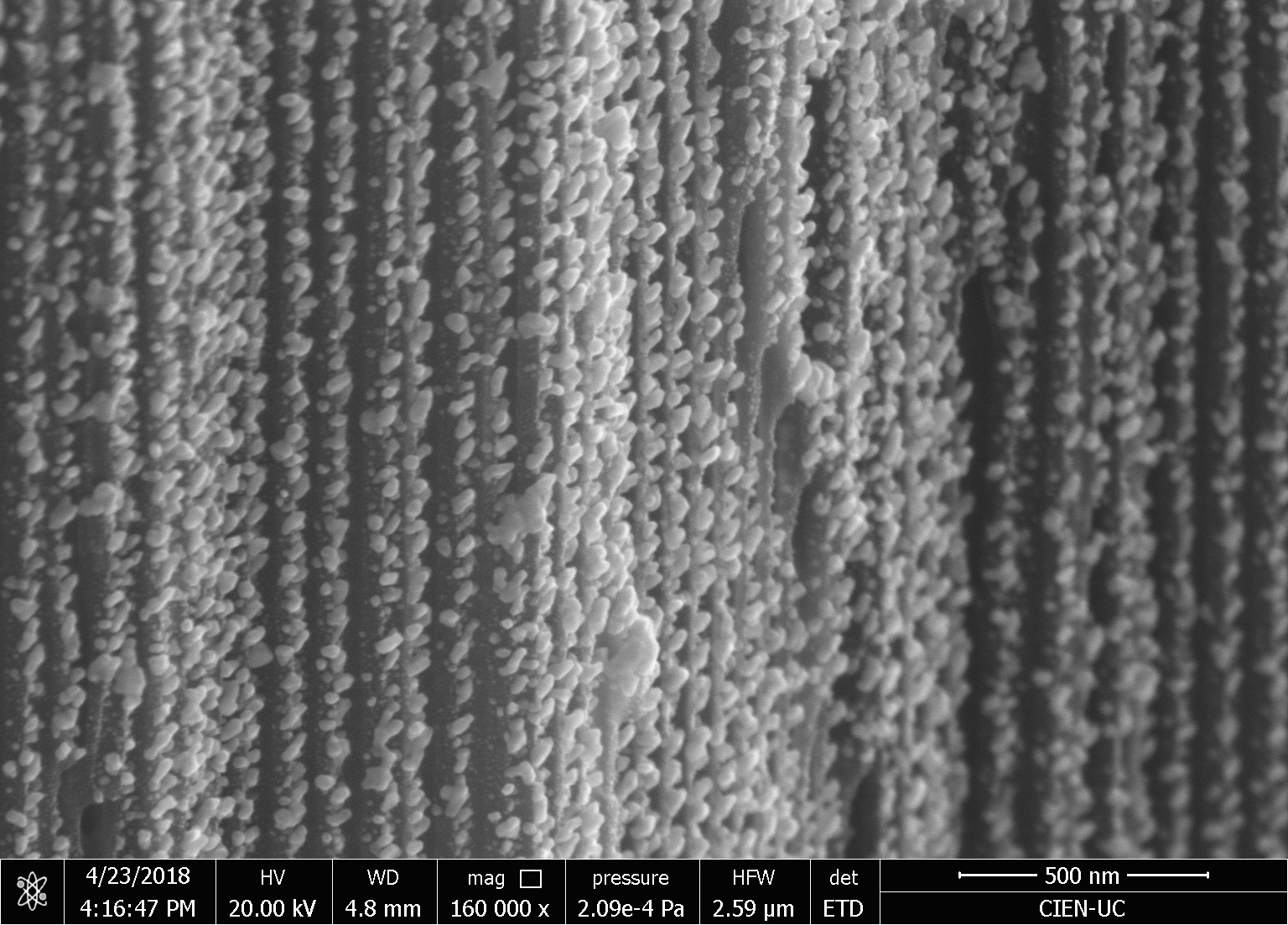
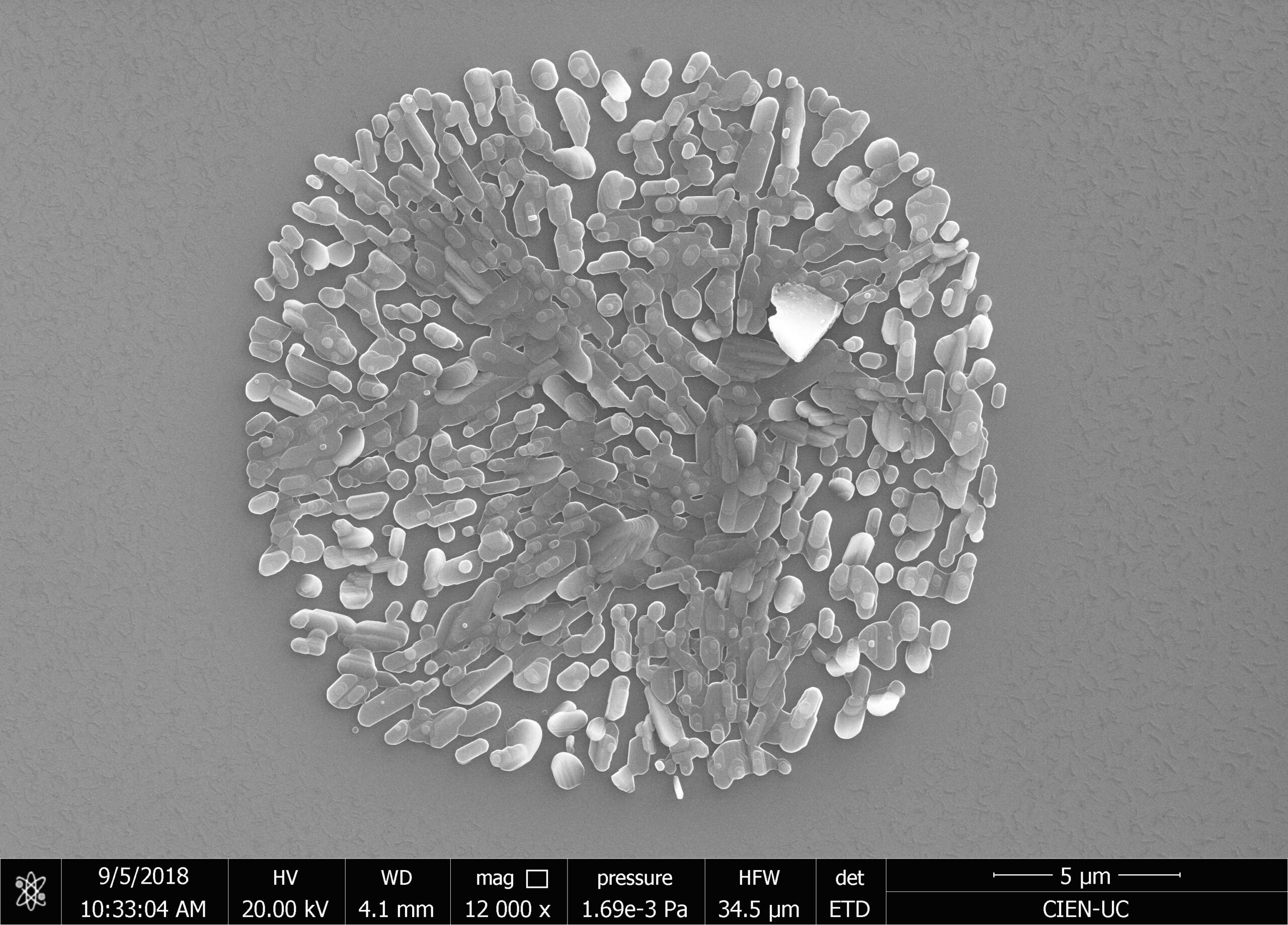
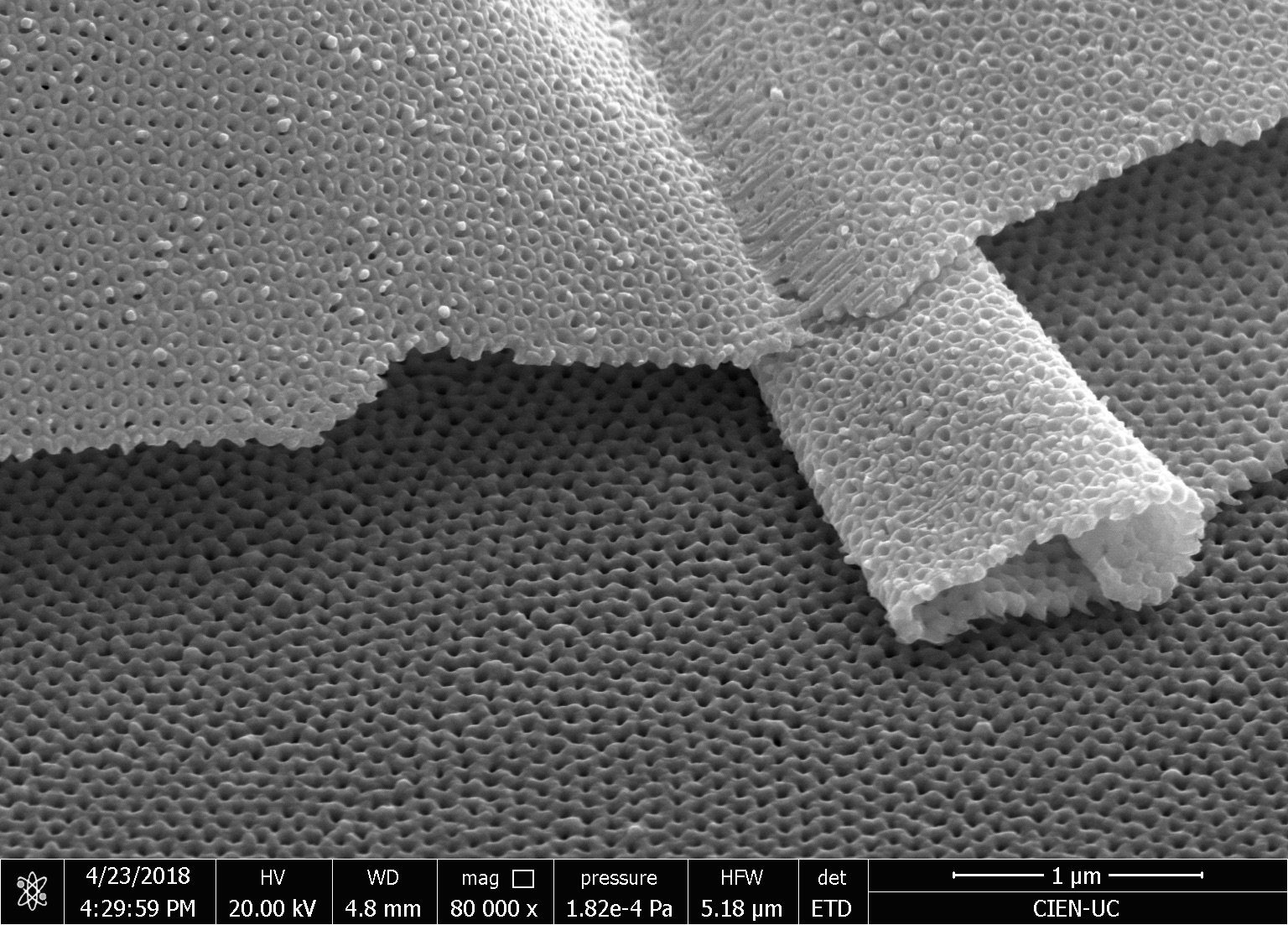
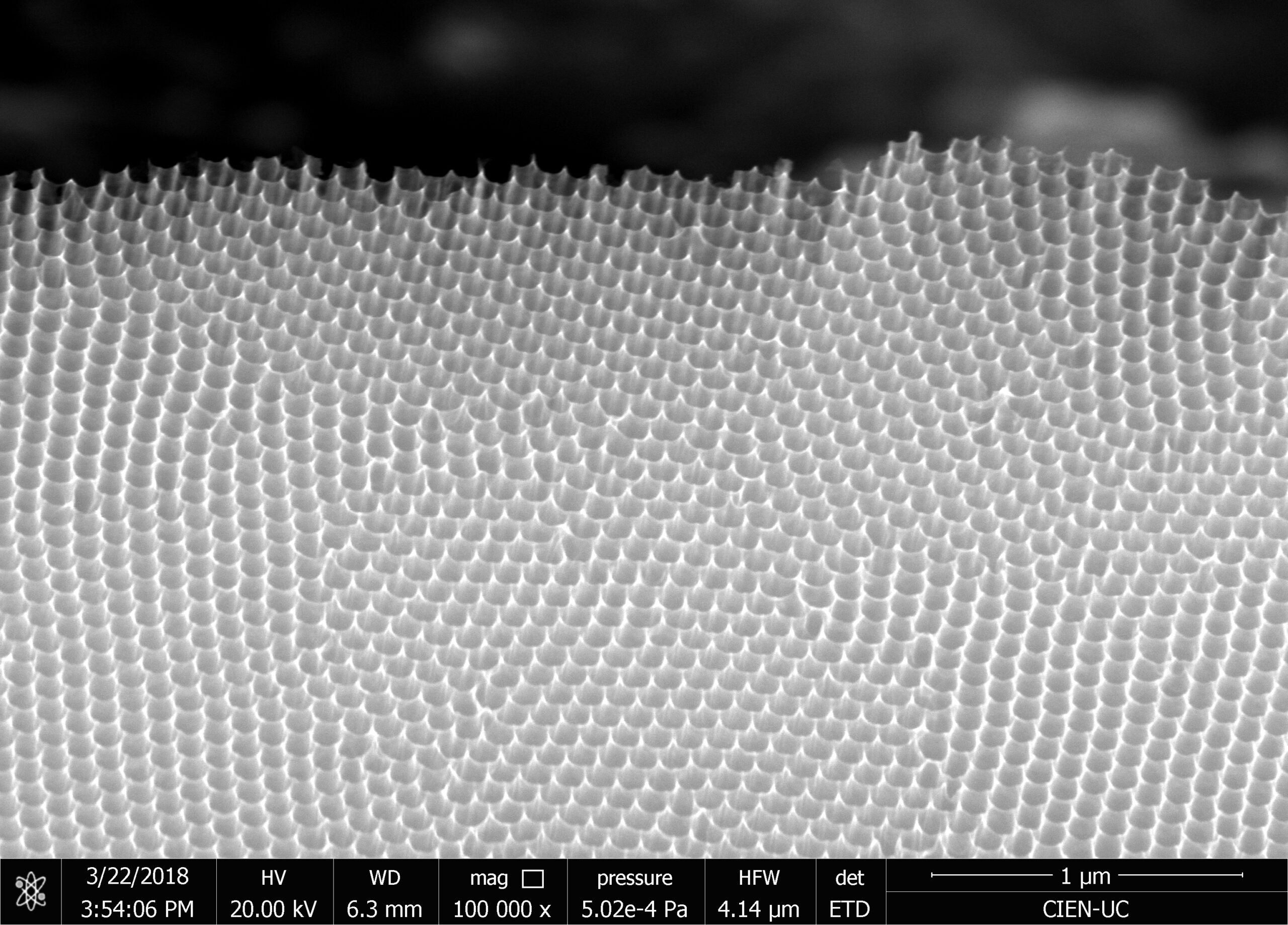
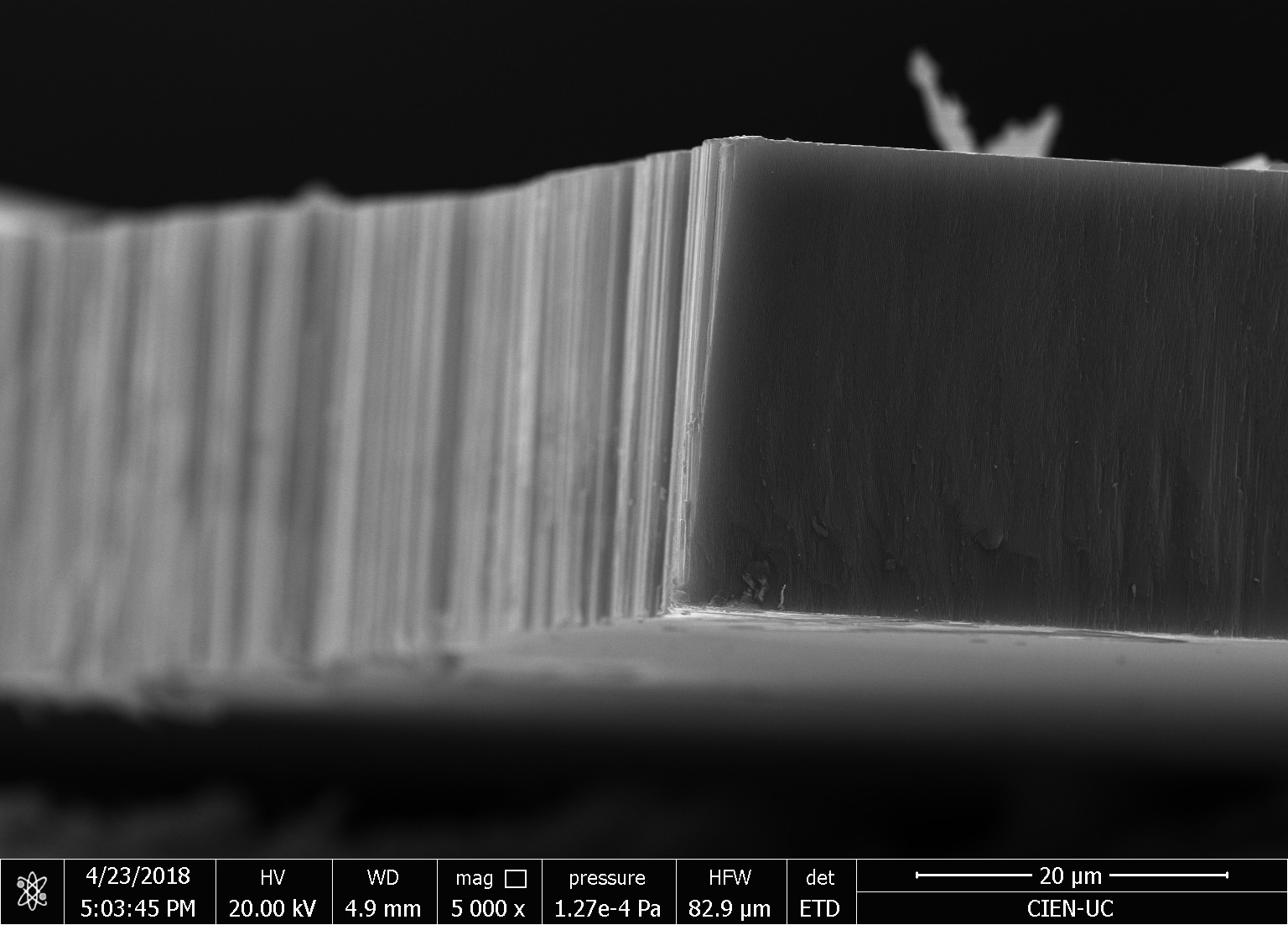
Our research consists in explore fabrication methods of nanomaterials and study their physical and chemical properties using advanced experimental techniques. The objective is to develop nanomaterials to use in technological applications. Particularly, we study how to employ them to assembling efficient photocatalysts for hydrogen production using sunlight, in cathodes for lithium-ion batteries, and also in chemical and biological sensors. One of the ways to manufacture these nanostructures is by using a nanoporous membrane synthesized in a controlled way, such as is possible to modulate their pore size from 20 nm to 200 nm and their thickness from 100 nm to 100 um. We can make nanotubes, nanorods, and nanodots of different materials, such as carbon nanotubes, nickel nanorods, vanadium dots, etc. We use several techniques of microscopy and spectroscopy to characterize these materials and their properties, such as electric transport properties at low temperatures (up to -260 ºC).
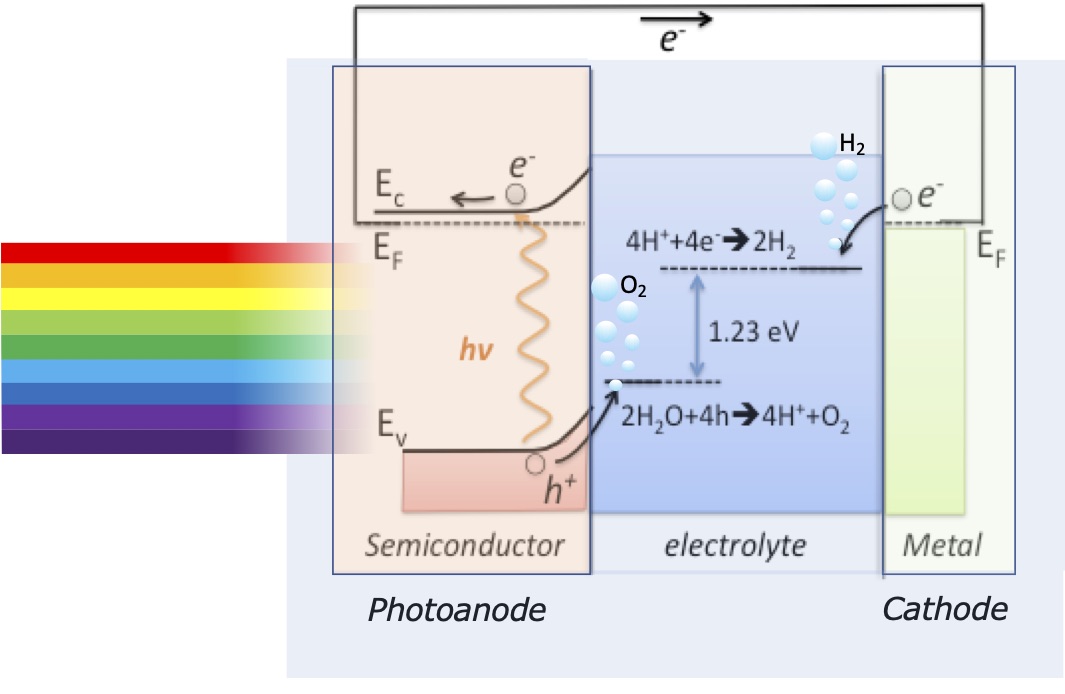
The efficient production of hydrogen by splitting water molecules through photocatalysts using solar energy is one of the most important challenges of Energy Sciences nowadays. Water and sunlight are both renewable, abundant, and inexpensive sources. In fact, the amount of solar power, about 95000 TW average, that striking the whole of earth’s surface is more than 5000 times higher than the current power needs for our civilization. Particularly, Chile hosts some of the sunniest places on earth, which has led to the growing solar energy industry in recent years. For its part, the Chilean government has sponsored several initiatives to promote the use of solar energy. Therefore, the development of research in this topic is highly pertinent for our country, not only for their scientific relevance and potential application, but also for their contribution to the advanced human capital formation needed for the local development of these technologies.
The main goal of our research is to fabricate a photoelectrochemical cell to produce hydrogen by water splitting using a dual absorber tandem cell. We explore photoelectrodes fabricated mainly of transition metal oxides of earth-abundant materials, such as TiO2, Fe2O3, Cu2O, and also perovskites. Searching to improve their performance through nanostructuring and combining with dissimilar material such as metal nanoparticles and carbon nanotubes, in a novel configuration. To achieve this goal, we will combine physical and chemical fabrication methods in a synergic way, in order to grow the respective material with the appropriate technique.
Our research on lithium-ion batteries (LiB), relies on searching for new materials to develop Cathode and Anodes that allow high-voltage and high-capacity LiBs.
As members of MultiMat, we develop a simple fabrication route to prepare V2O5 films that consists of the evaporation by electron-beam of vanadium films followed by an ex-situ oxidation. The V2O5 films display optimum capacity, close to the theoretical value with an excellent rate capability. These characteristics strongly depend on the initial metal thickness and oxidation temperature. This oxide is a model system as it is a cathode material that works without the need for carbon additives.
Part of our collaboration consists of SEI investigations using the self-assembled molecules approach (SAMs). SAMs are intended to protect the cathode against degradation and to promote, in a controllable manner, the formation of the desired SEI on these model surfaces. Also, we work in the computational modeling of V2O5 crystal structure using density functional theory and quantum molecular dynamics, being able to predict the voltage profile for lithium intercalation.

The detection of volatile compounds is an important challenge in several applications nowadays. Air pollution analysis, explosive detection, food quality and pesticides control are some of many cases that require specific sensors. For example, in the context of a hydrogen-based energy economy, the development of hydrogen sensors capable to achieve high sensitivity at low detection limits is particularly relevant. Low-cost microelectronic gas sensors based on materials as piezoelectric quartz, metal oxides or conducting polymers have been fabricated, achieving remarkable sensitivities, but lacking in adequate selectivity, which is a crucial requirement in some cases. High sensitivity detection can be reached by using nanoscale structures, such as nanoporous materials, nanotubes and graphene.
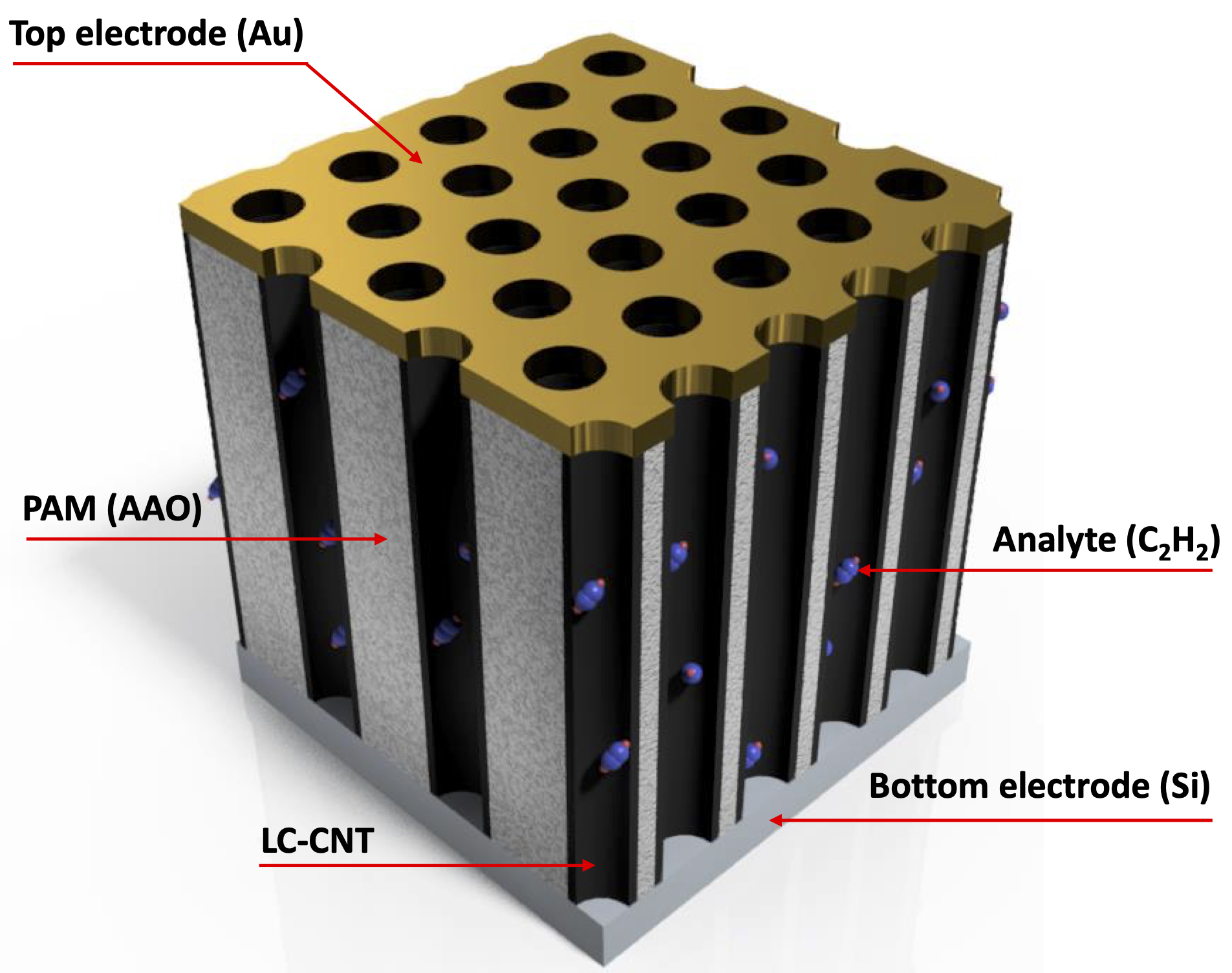
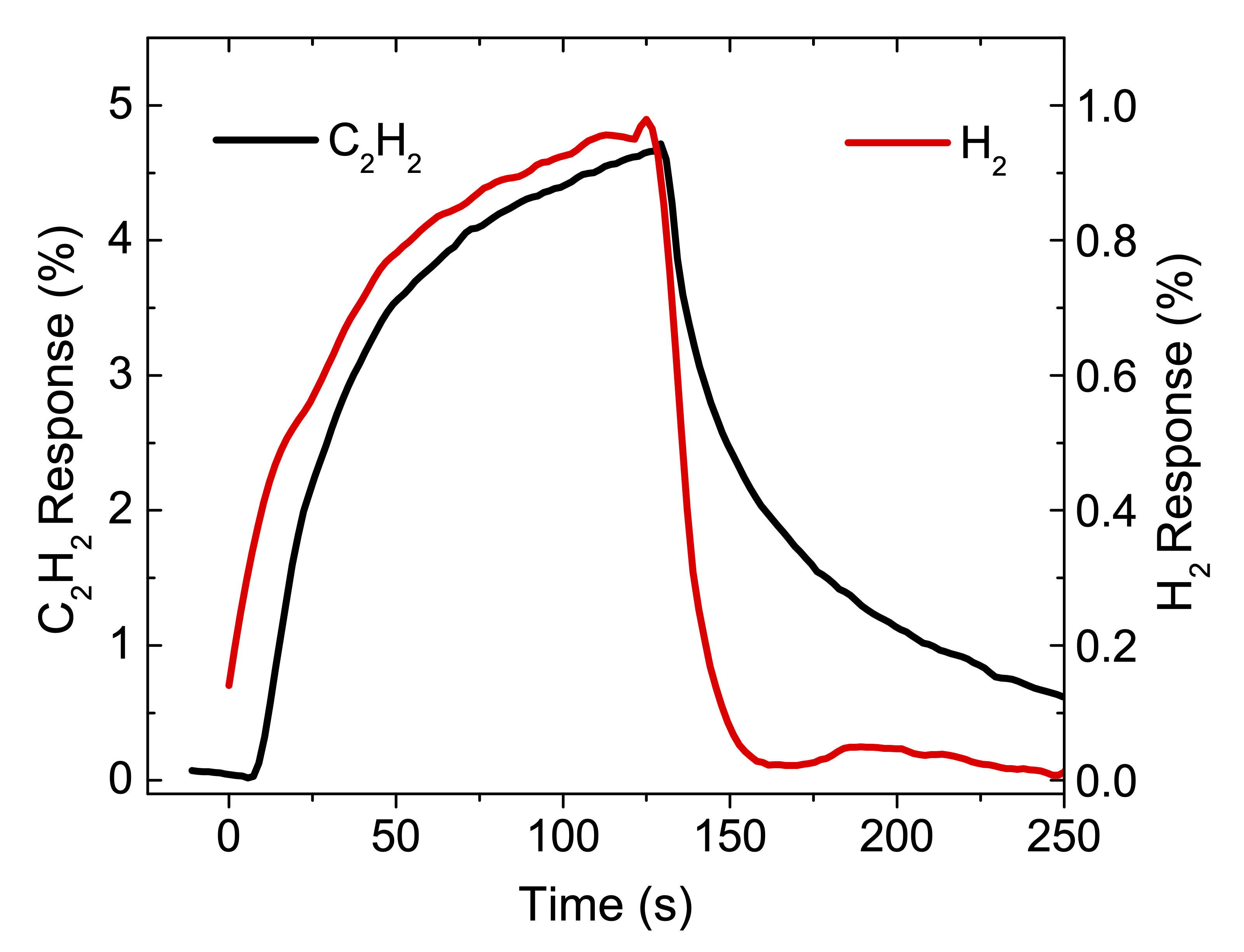
We investigate the performance as a gas sensor of a device based on low crystallinity carbon nanotubes (LC-CNT) supported by porous alumina membranes (PAM), operating at room temperature under argon atmosphere. A scheme of a device is shown in the Figure. In order to evaluate the performance of this material as gas sensor, several devices containing LC-CNTs with different wall thickness were prepared. The devices were exposed to H2 and C2H2 under Ar atmosphere to explore the resistive response by applying voltage between top (gold) and bottom (N-doped Si) electrodes and measuring the electric current through the nanotubes array. The maximum observed resistive responses are similar to other sensors based on non-doped CNT, however in this case they showed a remarkable quick response (few seconds) and short recovery time (few minutes) at room temperature.